Molecular sieve, also named synthetic zeolite, is a crystal in silica aluminates with multiple micro pores. It is a basic skeleton structure formed by silicon to oxygen and aluminum to oxygen tetrahedron. In its lattice, there are metal positive ions, such as sodium ion, potassium ion, calcium ion and more, which can balance the excess negative charges. According to its crystal structure, molecular sieve can be mainly classified as A type, X type, Y type and so on.
| The chemical formula of the zeolite unit cell | Mx/n [ (AlO2)x (SiO2)y ]WH2O |
| Mx/n | Positive ion, Keep the crystal neutral |
| (AlO2)x(SiO2)y | The skeleton of the zeolite crystals, Possess different shapes of holes and pore canals |
| H2O | Chemical adsorption and physical adsorption of water molecules, Occur reversible desorption and adsorption under certain conditions |
Adsorption function
Molecular sieve adsorption of substances originates from physical adsorption called van der Waals force. There are extremely strong polarity and the Coulomb field in the crystal cavity. Also, it expresses strong adsorption capacity for unsaturated molecules and polar molecules like water.
Sieving function
Distribution of zeolite pore size is very homogeneous. Only substance of which molecular diameter is smaller than aperture diameter has the possibility to enter the internal bug hole of molecular sieve. It distinguishes the molecules of different substances by priority of adsorption and size dimension, so it is vividly called molecular sieve.
A type molecular sieve
 A type molecular sieve is mainly composed of silica aluminates, which is LTA type skeleton structure formed by silicon to oxygen and aluminum to oxygen tetrahedron with the main crystal pore of eight membered ring structure.
A type molecular sieve is mainly composed of silica aluminates, which is LTA type skeleton structure formed by silicon to oxygen and aluminum to oxygen tetrahedron with the main crystal pore of eight membered ring structure.
The main crystal pore diameter is 4 angstrom or 0.4 nm, which is called 4A type or sodium A type molecular sieve.
Calcium ion that exchanges sodium ion of 4A type molecular sieve forms 5 angstrom of pore diameter, which is called 5A type or calcium A type molecular sieve.
Potassium ion exchanging sodium ion of 4A molecular sieve forms 3 angstrom of pore size, which is named 3A type or potassium A type molecular sieve.
HADsiev™ (3A)|| HADsiev™ (3A EPG)
HADsiev™(4A)|| HADsiev™(4A DG)|| HADsiev™ (5A)
X type molecular sieve
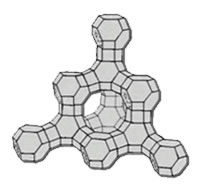 X type molecular sieve mainly consists of silica aluminates. Silicon to oxygen and aluminum to oxygen tetrahedron forms FAU type skeleton structure, and its main crystal pore structure is twelve-membered ring.
X type molecular sieve mainly consists of silica aluminates. Silicon to oxygen and aluminum to oxygen tetrahedron forms FAU type skeleton structure, and its main crystal pore structure is twelve-membered ring.
Different crystal structure or distinct silica alumina ratio can form molecular sieve crystal with the pore size of 9 to 10 angstrom, called 13X or sodium X type molecular sieve.
Calcium ion exchanging sodium ion of 13X molecular sieve can form the molecular sieve crystal with the pore diameter of 8 to 9 angstrom, which is named 10X or calcium X type molecular sieve.
HADsiev™10A (13X)|| HADsiev™ (13X APG)
HADsiev™(13X HP)|| HADsiev™ (X-HP-3)|| HADsiev™(10X)

