The corresponding crystallographic structure is formed by tetrahedras of AlO4 and SiO4. These tetrahedras are the basic building blocks for various zeolite structures, such as zeolites A and X, the most common commercial adsorbents.
 |  |
| Molecular Sieve Type A | Molecular Sieve Type X |
Due to the presence of alumina, zeolites exhibit a negatively charged framework, which is counter-balanced by positive cations resulting in a strong electrostatic field on the internal surface. These cations can be exchanged to fine-tune the pore size or the adsorption characteristics. For instance, the sodium form of zeolite A has a pore opening of approximately 4 angstrom or 0.4 nm, called 4A molecular sieve. If the sodium ion is exchanged with the larger potassium ion, the pore opening is reduced to approximately 3 angstrom, named 3A molecular sieve. On ion exchange with calcium, one calcium ion replaces two sodium ions. Thus, the pore opening increases to approximately 5 angstrom, called 5A Molecular Sieve. Ion exchange with other cations is sometimes used for particular separation purposes.
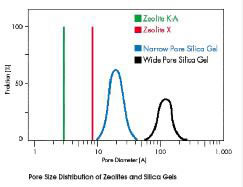 |
| The pore opening of the sodium form of zeolite X (13X) is approximately 9 to 10 angstrom. |
Of note is the high capacity of Molecular Sieves of Running Engineering even at low water concentration, allowing to dry very low water contents. Running HADsiev® molecular sieve can retain its high capacity at high temperature, which makes it the optimal material if drying needs to be carried out at comparatively high temperatures.
The adsorption process is fully reversible and of purely physical nature. The structure of the zeolite stays intact during the adsorption process and its later regeneration, and dissolution effects like with other drying agents like calcium compounds can not happen.

